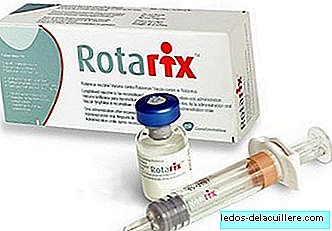
Rotarix is one of the vaccines marketed in Spain against rotavirus, an oral vaccine that protects against serotypes that are most often the cause of gastroenteritis in children and is recommended in vaccination schedules.
But nevertheless, The Rotarix brand will stop marketing in Spain because DNA residues of a virus called a virus have been found in the vaccine porcine circovirus 1 (PCV-1). New lots will not enter the market until more information about this finding is known.
This virus does not represent a health problem, as it does not cause pathology in humans or animals, as stated by the World Health Organization (WHO), the European Medicines Agency (EMA) or the Food and Drug Administration of the United States (FDA).
The WHO notes that no change in the administration of the Rotarix vaccine is necessary. The EMA in its latest press release on the matter notes that it sees no health risks and does not consider the restriction of vaccine use necessary.
However, as announced by the FDA at this time, it is recommendedto suspension in the United States the administration of this vaccine until more data is obtained on the safety or not of these virus fragments that should not be in the Rotarix.
This same address follows the recommendations in Spain of the Spanish Association of Pediatrics. The non-entry into the market of new lots of Rotarix implies the shortage in pharmacies of the product, although we do not know that the sale of existing lots has been prohibited.
In Spain, 2 vaccines have been marketed that aim to act against rotavirus infection that causes gastroenteritis: Rotarix de GlaxoSmithKline (GSK) and RotaTeq by Sanofi Pasteur MSD. After the restriction of the lots of Rotarix, the AEP recommends the administration of the other brand, RotaTeq.
We see the following in the joint informative note that you can read in full from the vaccination section of the Spanish Association of Pediatrics. It has been prepared by the Spanish Association of Pediatrics (AEP), the Spanish Association of Vacunology (AEV) and the Spanish Society of Pediatric Infectology (SEIP):
Although WHO and the EMA have established that there is no reason to limit the use of Rotarix® and therefore do not recommend any change in the use of the vaccine, AEMPS has decided as a precaution, since these viral fragments of PCV -1 should not be in this vaccine, do not authorize the release of new lots of Rotarix® vaccine to the Spanish market. This decision will cause in the next few days or weeks a shortage of this vaccine in the pharmaceutical distribution channel. For this reason, and waiting for GSK to provide additional information and resolve this situation, AEMPS has established a recommendation not to initiate new vaccination guidelines with Rotarix® in Spain at this time, advising the use of an alternative vaccine (RotaTeq® ) for vaccination against rotavirus. AEMPS also raises for children who have only received one dose of Rotarix® the possibility of completing vaccination with two doses of RotaTeq®.
As we see, there is no reason for the alarm in case your children have this vaccine.
However, it is normal for news such as finding a virus that should not be in a vaccine and Rotarix withdrawal leave us parents with a sense of insecurity and some concern.